Resource: Facts About COVID-19 Vaccines
What are the Different Types of Vaccines?
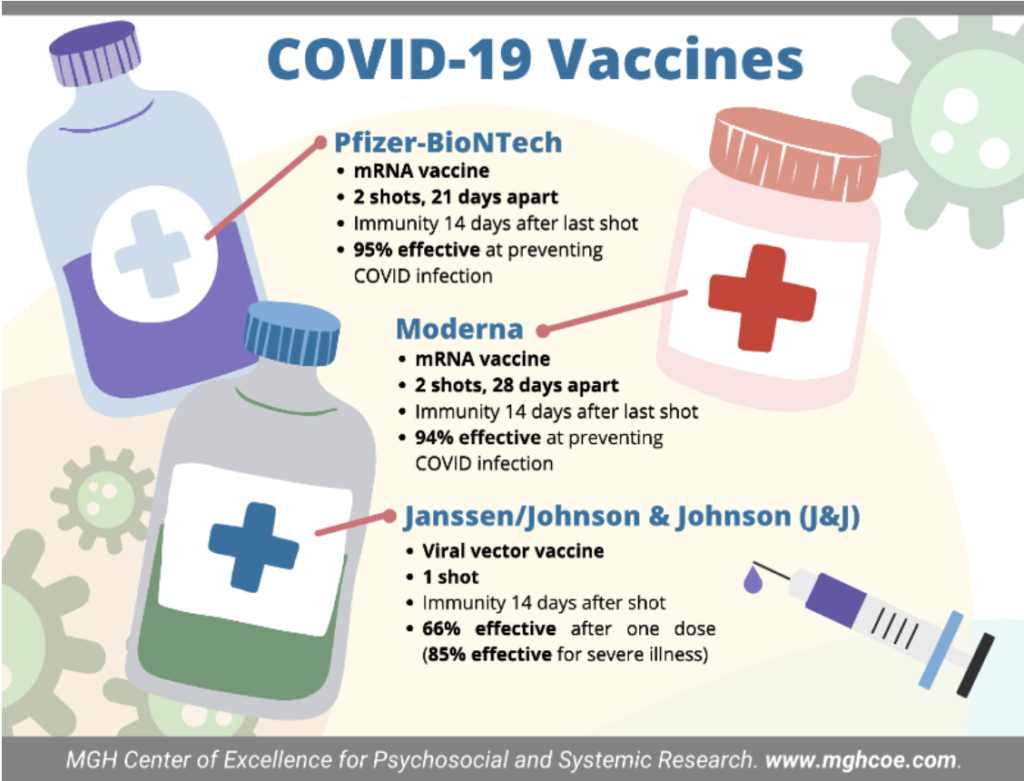
Pfizer-BioNTech, Moderna, and Johnson & Johnson have all developed an FDA approved vaccine for COVID-19. Read on to learn specifics and differences about the vaccines.

Pfizer-BioNTech
Q: What kind of vaccine is this?
A: This vaccine uses a technology that delivers messenger RNA (mRNA), a bit of genetic code that is like a recipe, to cells to make a protein unique to the COVID-19 virus (the so-called “spike” protein on the surface of the virus). The protein triggers an immune response which teaches your body to see the spike protein as foreign and develop antibodies to fight the COVID-19 virus if you become infected in the future. The mRNA from the vaccine never enters the nucleus of the cell (where our DNA is kept) so it does not interact with your DNA nor get integrated into your DNA. See Infographic C below.
Q: How is it delivered?
A: Two shots, 21 days apart.
Q: When is a person immune?
Immunity 14 days after the second shot.
Q: How effective is it?
A: 95% at preventing symptomatic COVID infection in those with no evidence of previous COVID-19 infection. Pfizer/BioNTech vaccine is considered effective against the South African variant.

Moderna
Q: What kind of vaccine is this?
A: Made using messenger RNA (mRNA), like the Pfizer-BioNTech vaccine
Q: How is it delivered?
A: Two shots, 28 days apart
Q: When is a person immune?
A: Immunity 14 days after the second shot
Q: How effective is it?
A: Efficacy at 94%. Trials have begun to examine effectiveness against variants.

Janssen/Johnson & Johnson (J&J)
Q: What kind of vaccine is this?
A: Viral vector vaccine: this vaccine uses a modified version of a different, harmless virus (acting as the “vector”) which enters a cell and makes a harmless piece of the COVID-19 spike protein (not a full virus). Your immune system recognizes the spike protein and builds antibodies that protect you against COVID-19 in the future. J&J employed this same approach to make an Ebola vaccine that has been authorized for use.
Q: How is it delivered?
A: One shot
Q: When is a person immune?
A: Immunity 14 days after the shot
Q: How effective is it?
A: Compared to Pfizer and Moderna, the effectiveness of the J&J vaccine was measured slightly differently as this aimed to test whether the vaccine protected against moderate-severe COVID illness (a positive COVID test and at least one symptom) and was tested after the emergence of the new variants (from Britain, South Africa, and Brazil). The vaccine was shown to be 66% protective after one dose (85% effective for severe illness).
Infographic B. Quick Overview of COVID-19 Vaccines. Click here to view full size.

Infographic C. CDC Guide: How mRNA COVID-19 Vaccines Work.
Click here to view full size.
Side Effects:
A COVID-19 vaccine can cause mild side effects after the first or second dose, including:
- Pain, redness, or swelling where the shot was given
- Fever
- Fatigue
- Headache
- Muscle or joint pain
- Nausea and vomiting
- Swollen lymph nodes
You will be monitored for 15 minutes after getting a COVID-19 vaccine to see if you have an immediate reaction. Most side effects happen within the first three days after vaccination and typically last only one to two days. These are signs of your immune system being stimulated and although unpleasant, show that the vaccine is working. If symptoms persist beyond two days, you should call your doctor.
How are the vaccines being distributed?
In Massachusetts:
- Phase 1 (December-February): Healthcare workers, long-term care facilities, rest homes, assisted living facilities, first responders, and care settings staff.
- Phase 2 (Eligible now): People who are 60 and over, those with two or more medical conditions, people who live/work in low income and affordable senior housing, K-12 staff, and childcare workers.
- Eligible April 5th: People 55 and over and those with one medical condition.
- Phase 3 (Eligible on April 19th): Anyone 16 years or older who is not listed in Phase 1 or 2.
Check if you are eligible now: https://www.mass.gov/covid-19-vaccine
Q: Who is eligible on April 19, 2021?
A: On April 19, 2021, Patriots’ Day, vaccination appointments will be opened to all Massachusetts residents 16 years or older.
Q: How do I sign up?
A: All Massachusetts residents can now pre-register for a COVID-19 vaccination appointment at one of the state’s vaccination sites. Visit https://www.mass.gov/info-details/preregister-for-a-covid-19-vaccine-appointment to pre-register.

Frequently Asked Questions
Gathered from CDC (1), Massachusetts General Hospital (3), and Johns Hopkins (4).
Q: Can a COVID-19 vaccine make me sick with COVID-19?
A: No. None of the authorized vaccines contain the live virus that causes COVID-19. As noted, the different vaccines that are available in the US teach the immune system to recognize and fight the virus. Sometimes this process can cause symptoms, such as fever or fatigue, and these symptoms are normal and a sign that the body is building protection against the virus.
Q: If I have already had COVID-19, do I still need to get vaccinated?
A: Yes. You should be vaccinated regardless of whether or not you have had COVID-19. Experts do not know how long you are protected from getting sick again after recovering from COVID-19 nor how common it is for individuals to be reinfected with COVID-19 again. Also, COVID-19 vaccinations are an important tool to help stop the pandemic. Wearing masks and social distancing help reduce the chances of being exposed or spreading the virus, but these measures are not enough. Vaccines work with your immune system to be ready to fight the virus if you are exposed.
Q: Will a COVID-19 vaccination protect me from getting sick with COVID-19?
A: Yes. COVID-19 vaccination works by teaching your immune system how to recognize and fight the virus that causes COVID-19, and this protects you from getting sick with COVID-19.
Q: Will a COVID-19 vaccine alter my DNA?
A: No. The COVID-19 vaccines do not change or interact with your DNA (see description of how the vaccines work above)
Q: Is one of the COVID-19 vaccines better than the other? Will I have a choice of which vaccine I receive?
A: No. All three vaccines have been shown to be effective at preventing severe illness, hospitalization, and death. All vaccines are currently being shipped across the US and people will not have the option to select which vaccine they receive. Once you receive your first shot, the second shot will be from the same manufacturer, e.g., if you receive a first dose of the Pfizer/BioNTech vaccine, your second dose will also be of the Pfizer/BioNTech vaccine.
Q: If I get the vaccine, do I still need to wear a mask and practice social distancing?
A: Yes. Vaccines do not stop the coronavirus from entering your body and perhaps from you spreading the virus without noticing it; they only prevent you from developing moderate to severe COVID-19. At this stage, there is not enough information available to determine when the CDC will no longer recommend masks or physical distancing.
Q: I am pregnant. Should I get the COVID-19 vaccine?
A: Vaccinations, such as the flu shot, are considered a safe and routine part of prenatal care. It is recommended that you speak with your OB care providers about the COVID-19 vaccine. Pregnant people were not included in the trials of the COVID-19 vaccine, so safety data is limited. More than 30,000 pregnant individuals have received the Pfizer/BioNTech or Moderna vaccines in the U.S. since December 2020 and the CDC reports that there are no safety concerns.
See MGH website for more info: https://www.massgeneral.org/obgyn/news/COVID-19-Vaccine-FAQ-for-Pregnant-and-Breastfeeding-People
Q: Is it safe for me to get a COVID-19 vaccine if I would like to have a baby one day?
A: Yes. There is currently no evidence that COVID-19 vaccination causes any problems with pregnancy, including the development of the placenta, nor fertility problems
Q: Did researchers rush the development of the vaccine? Can the effectiveness and safety be trusted?
A: The vaccines have been shown to be highly effective with no serious or life-threatening side effects. The vaccine could be developed so quickly because:
- The COVID-19 vaccines from Pfizer/BioNTech and Moderna were created with a method that has been in development for many years, so the companies could start the vaccine development process early in the pandemic.
- China isolated and shared genetic information about COVID-19 promptly, so scientists could start working on vaccines.
- The vaccine developers didn’t skip any testing steps but conducted some of the steps on an overlapping schedule.
- Vaccine projects had plenty of resources, as governments invested in this research.
- Some types of COVID-19 vaccines were created using messenger RNA (mRNA), which allows a faster approach than the traditional way that vaccines are made.
- Social media helped companies find and engage study volunteers.
- Because COVID-19 is so contagious and widespread, it did not take long to see if the vaccine worked for the study volunteers who were vaccinated.
- Companies began making vaccines early in the process before it was clear if they would even work, so some supplies were ready when FDA approval was granted.
Q: Was the COVID-19 vaccine developed with controversial substances?
A: The first two COVID-19 vaccines to be authorized by the FDA (Pfizer/BioNTech and Moderna) contain mRNA and other, normal vaccine ingredients, such as fats (which protect the mRNA), salts, as well as sugar. These COVID-19 vaccines were not developed using fetal tissue, and they do not contain implants, microchips, or tracking devices.

Abigail Wright, PhD
Research Staff & Junior Investigator, MGH COE
Twitter: @DrAbigailW
https://www.mghcoe.com/
References
- Center for Disease Control and Prevention. (2021). Myths and Facts about COVID-19 Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/facts.html
- Center for Disease Control and Prevention. (2021). Understanding mRNA COVID-19 Vaccines. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/mrna.html
- Massachusetts General Hospital. (2021). COVID-19 Vaccine. https://www.massgeneral.org/news/coronavirus/vaccine
- John Hopkins Medicine. (2021). COVID-19 Vaccines: Myth Versus Fact. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/covid-19-vaccines-myth-versus-fact